LAJU REAKSI
•CONCENTRATION OF SOLUTION
• MIXTURE CONCENTRATION
• RATE CONCEPT
• FACTORS OF RATE
• HOW TO DETERMINE REACTION RATE
ACCORDING TO DATA OF FIRST CONCENTRATION
KONSENTRASI LARUTAN
Molar consentration
is amount of moles solute in1 liter solution. Formula of molar concentration is :
gr 1000
M = mol/V M = ----------- x -------------
Mr vol
Problem
:
1.
Determine molar concentration of solution 2
mol NaOH in 100 mL water
2.
Determine molar concentration of 6
gram urea solubled in
water so volume 250 mL.
3.
Determine molar concentration of H2SO4 (p), if on the botle label
writen that presentation H2SO4 is 96% and density of solution is
1,8 Kg/L.
4.
Student will make 1 litre of ureum solution 1 M.How much mass of ureum
needed.
PENGENCERAN LARUTAN
PENCAMPURAN LARUTAN
LAJU REAKSI
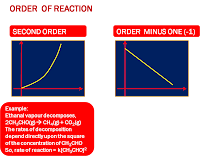
ORDE REAKSI
FAKTOR-FAKTOR LAJU REAKSI
KATALIS










Tidak ada komentar:
Posting Komentar